Isotopes and Specific Charge
Isotopes and Specific Charge Revision
Isotopes and Specific Charge
In nature, elements do not all have the same constituents. While maintaining the same number of protons, elements can vary in the number of neutrons they possess. These are isotopes of the element.
Isotopes
Atoms with the same number of protons but a different number of neutrons are called isotopes. The number of electrons remains constant.
There is no change in the chemical properties of an atom when the number of neutrons are changed. However, the nucleon number of the atom will change.
The stability of the nucleus is changed when the neutron number is changed. An unstable nucleus can lead to radioactive decay. When a nucleus is unstable, it continually decays, releasing radiation and forming new, more stable nuclei. This can occur over a range of timescales, from seconds to thousands of years.
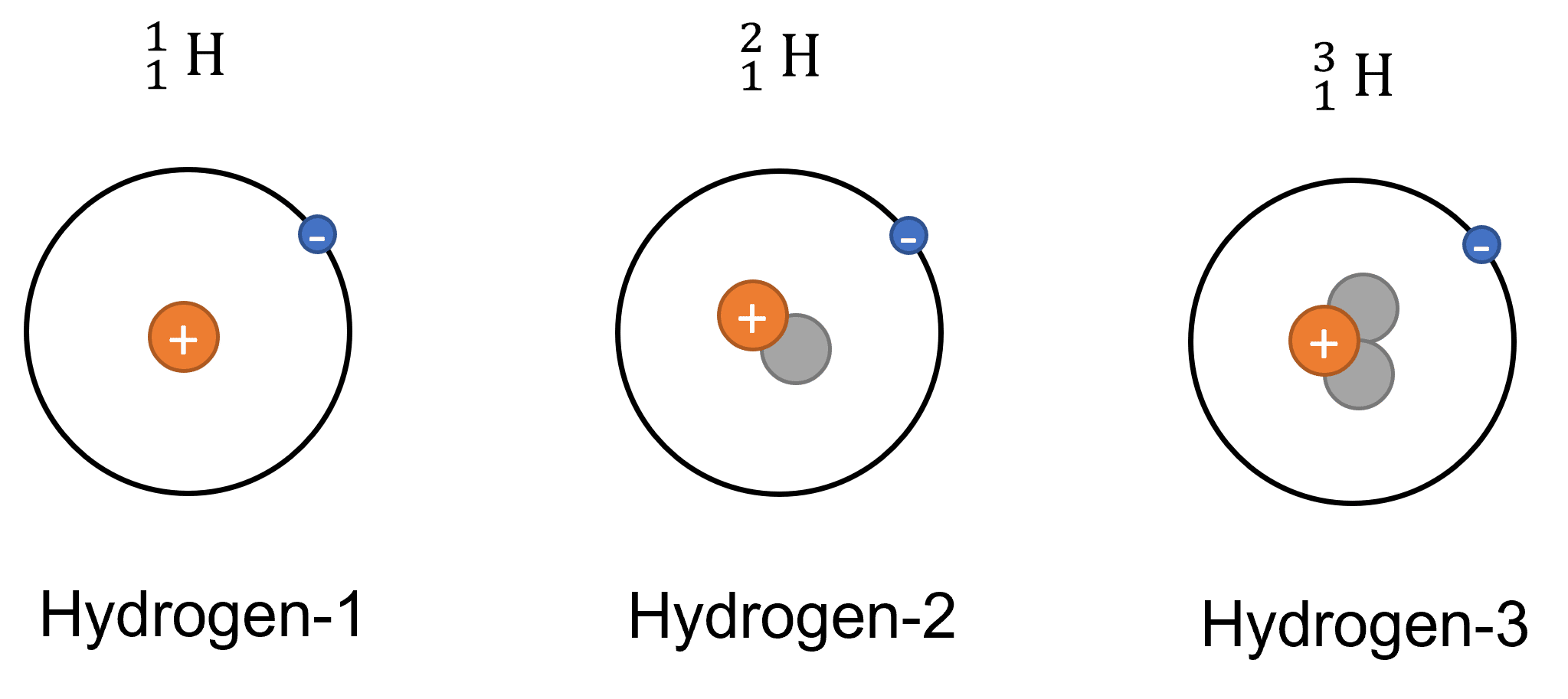
The example above shows three natural isotopes of Hydrogen. Hydrogen has 1 proton and 0 neutrons. Deuterium (Hydrogen-2) has 1 proton and 1 neutron. Tritium (Hydrogen-3) has 1 proton and 2 neutrons.
Carbon-14 Dating
Isotropic data is the relative amount of each isotope of an element found in a substance.
It helps formulate an isotropic signature, which is a ratio of the abundance of isotopes in a substance.
Isotropic data is used to determine the age of organic and inorganic materials through radioactive dating, with Carbon-14 dating being one example.
Carbon-14 is a naturally occurring isotope of Carbon, containing 6 protons and 8 neutrons. It is present in all living objects and radioactively decays. It decays via beta decay to nitrogen-14. In plants and animals, Carbon-14 is continually replenished and the ratio between Carbon-12 and Carbon-14 remains constant.
When an animal or plant dies, Carbon-14 is no longer replenished and the amount begins to decline as it decays to more stable elements. Carbon-14 has a half life of around 6000 years. Therefore the age of the material can be determined by be determined by looking at the Carbon-14 abundance and using the half life.
Specific Charge
The specific charge of a particle is a ratio of its charge to its mass. It is measured in coulombs per kilogram\left(\text{C kg}^{-1}\right).
\textcolor{00bfa8}{\text{Specific Charge} = \dfrac{\text{Charge}}{\text{Mass}}}
The specific charge of an element is always 0, as the charge is 0 (Proton number = Electron number).
You could be expected to find the specific charge of any particle, such as a fundamental particle, nucleus of an atom or an ion.
The specific charge of an electron and proton are in the data sheet:
- Electron charge/mass ratio – 1.76\times 10^{11} \: \text{C kg}^{-1}
- Proton charge/mass ratio – 9.58 \times 10^7 \: \text{C kg}^{-1}
You may need to use the actual charge and masses of protons, neutrons and electrons, which are all given in the data sheet.
The following equation can be used to find the mass of a nucleus or ion:
\textcolor{00bfa8}{\text{Mass} = \text{Number of Nucleons} \times 1.67 \times 10^{-27} \: \text{kg}}
To find the total charge of a nucleus, we use the total charge of the protons:
\textcolor{00bfa8}{\text{Nucleus Charge} = \text{Number of Protons} \times 1.60 \times 10^{-19} \: \text{C}}
To find the total charge of an ion, we use the total number of electrons that have gained or lost:
\textcolor{00bfa8}{\text{Ion Charge} = \text{Number of electrons gained/lost} \times 1.60 \times 10^{-19} \: \text{C} }
Example: Calculating Specific Charge
An atom of \: _{\textcolor{7cb447}{40}}^{\textcolor{bd0000}{20}}\text{Ca} loses \textcolor{2730e9}{2} electrons. What is the specific charge of the ion?
[3 marks]
- First we need to find the number of protons and nucleons:
\text{Number of protons} = \textcolor{bd0000}{20}
\text{Number of nucleons} = \textcolor{7cb447}{40}
- Then we determine the overall mass of the ion:
\text{Mass of ion} = \textcolor{7cb447}{40} \times 1.67 \times 10^{-27} = 6.68 \times 10^{-26}
- For an ion, we determine the number of electrons that have been lost or gained:
\textcolor{2730e9}{2} electrons are lost from the element.
- Find the total charge that has been lost or gained:
\text{Ion Charge} = \textcolor{2730e9}{2} \times 1.60 \times 10^{-19} = 3.20 \times 10^{-19} \: \text{C}
- Substitute values into specific charge question:
\textbf{Specific Charge} = \dfrac{\text{Charge}}{\text{Mass}} = \dfrac{3.20 \times 10^{-19}}{6.68 \times 10^{-26}} = \boldsymbol{4.79 \times 10^6} \: \textbf{C kg}^{-1}
Isotopes and Specific Charge Example Questions
Question 1: State what is meant by an isotope.
[1 mark]
Atoms/elements with the same number of protons but different number of neutrons.
Question 2: How can Carbon-14 be used to find the age of a material?
[2 marks]
The amount of Carbon-14 remains stable in living organisms and only reduces once the organism dies.
Using the isotropic data to find the percentage remaining of Carbon-14 and using its half life, the age of the material can be used.
Question 3: Calculate the specific charge of a _{16}^{8} \text{O} nucleus.
[4 marks]








